Information Center
Discover the Power of Peptides
Welcome to the Leaf Hush Peptide Knowledge Center — your trusted guide to understanding the science, benefits, and uses of peptides in wellness and beauty. Explore curated insights, treatment overviews, and expert-backed resources to help you feel confident and informed. All articles and shared info are for educational purposes only
Understanding Peptide Bonds
Peptide bonds are the essential links that connect amino acids — the building blocks of life. These bonds are formed through a condensation reaction, where the carboxyl group of one amino acid combines with the amino group of another, releasing a molecule of water in the process. This bonding creates peptide chains, classified as: Dipeptides – 2 amino acids Oligopeptides – 10–20 amino acids Polypeptides – 50–100 amino acids Proteins – More than 100 amino acids Scientific studies, including X-ray diffraction, show that peptide bonds are rigid and planar, favoring a trans configuration due to steric hindrance (avoiding bulky clashes). Although these bonds appear single, they have resonance that limits rotation — helping maintain the structure and stability of proteins. To break a peptide bond, a process called hydrolysis occurs, which is crucial in protein breakdown and digestion.

Peptide Solubility
Peptide solubility depends on the amino acid composition and the overall charge of the peptide. When selecting a solvent, it's important to match it to the peptide’s characteristics. Peptides with high hydrophobicity dissolve best in organic solvents such as DMSO, methanol, or propanol. If the peptide is rich in acidic amino acids, basic solvents like ammonium hydroxide are more suitable. Conversely, peptides with a higher content of basic amino acids tend to dissolve better in acidic solvents such as acetic acid. Neutral peptides generally perform well in organic solvents like acetonitrile or isopropanol. Before preparing a full solution, it’s advisable to test solubility on a small sample and ensure the peptide has reached room temperature. Techniques such as gentle warming (below 40°C) or sonication can further aid in dissolution. Once fully dissolved, peptides should be slowly diluted into a buffered solution and stored at -20°C to maintain their stability and effectiveness.

Peptide Purification
Peptide purification is a crucial step in ensuring high-quality, research-grade peptides free from contaminants and byproducts. Depending on the peptide’s properties and the nature of impurities present, a variety of purification techniques may be used, including ion exchange chromatography (IEX), hydrophobic interaction chromatography (HIC), affinity chromatography (AC), reversed-phase chromatography (RPC), and gel filtration (GF). Each method targets specific impurities, such as hydrolysis products, deletion sequences, or polymeric forms of peptides, which can impact the purity and performance of the final product. Adhering to Good Manufacturing Practices (GMP) during purification ensures consistency, reproducibility, and compliance with regulatory standards—especially important for peptides intended for clinical or pharmaceutical use.
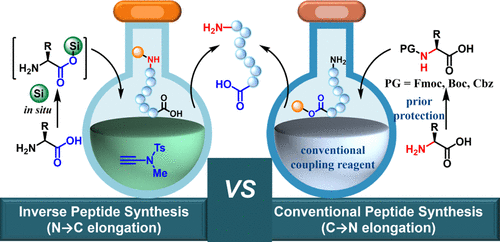
Peptide Synthesis
Peptide synthesis is a foundational process in peptide research and production, with Solid-Phase Peptide Synthesis (SPPS) being the most widely used method due to its high purity, yield, and efficiency. SPPS involves five repetitive steps: amino acid attachment, protection, coupling, deprotection, and finally, polymer removal. Each step is carefully controlled to ensure precise chain formation through C-to-N terminal linkage of amino acids. This method accommodates not only the 20 standard amino acids but also synthetic variants for specialized applications. The use of protecting groups—at the N-terminal, C-terminal, and sidechains—prevents unwanted side reactions, allowing accurate peptide assembly. Microwave-assisted SPPS is a modern enhancement that accelerates synthesis, although it may increase costs. After synthesis, techniques like Reverse-Phase Chromatography (RPC) and High-Performance Liquid Chromatography (HPLC) are employed to purify the peptide and eliminate residual impurities, ensuring high-quality final products for research or clinical use.

Standard Procedure for Storing Peptides
Proper storage is essential for preserving the stability and biological integrity of peptides. For long-term storage, peptides should be kept at -80°C (-112°F), while short-term refrigeration at 4°C (39°F) is suitable if the peptide will be used within days to a few months. It is critical to avoid repeated freeze-thaw cycles, which can lead to degradation. Peptides should be stored in airtight, chemically inert containers—with glass vials being ideal and polypropylene plastic vials as an acceptable alternative. To prevent moisture and oxidation, ensure the storage environment is dry and protected. For convenience and preservation, peptides should be aliquoted based on anticipated usage. When storing peptides in solution, sterile buffers with a pH between 5 and 6 are recommended to maintain stability and minimize breakdown.
Peptide Reconstitution
Peptides are typically supplied in lyophilized (freeze-dried) form, allowing for long-term stability. Before use, they must be reconstituted, and the choice of solvent depends on the peptide’s solubility profile, sequence characteristics, and application. Common solvents include sterile water or bacteriostatic water, although peptides with specific charges or properties may require different approaches. Basic peptides dissolve best in acidic solvents, while acidic peptides need basic solvents for optimal solubility. Hydrophobic or neutral peptides often perform well in organic solvents like DMSO, acetic acid, or propanol. However, care should be taken with peptides containing methionine or free cysteine, as they are prone to oxidation. Testing small samples and adjusting the solvent accordingly helps ensure full dissolution without compromising peptide integrity.
